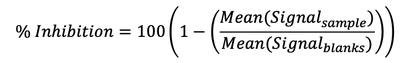Meso Scale Discovery (MSD®) Prototype Influenza 7-plex Serology Assay.
This assay protocol measures IgG antibody concentration in human plasma specific for Influenza vaccine hemagglutinin (HA) antigens: A/Brisbane, A/Hong Kong, A/Michigan, A/Victoria, B/Colorado, B/Phuket, and B/Washington. Briefly, MSD 96-Well 10-Spot multi-array plates coated with seven flu HA antigens were blocked, then human plasma samples diluted 10,000-fold, HA reference standards, and controls were added to the plate. Plates were shaken for 2 hours at 15°C to 25°C, washed, then anti-human IgG antibodies labelled with electrochemiluminescent (ECL) SULFO-TAG were added. Plates were shaken for an additional 1 hour at 15°C to 25°C, washed, then MSD GOLD Read Buffer B was added and the plates were read on an MSD SECTOR S600 ECL plate reader. Test samples were quantified in AU/mL referenced against a standard curve of HA-binding IgG reference standards.
Meso Scale Discovery (MSD®) 96-well hemagglutination inhibition (HAI) 9-plex Assay.
This hemagglutination inhibition (HAI) assay protocol measures neutralizing antibodies in human plasma that block the binding of labeled red blood cell vesicles to trimeric Influenza hemagglutinin (HA) antigens specific for the following lineages: A/Brisbane, A/Cambodia, A/Guangdong, A/HongKong, A/Kansas, A/Shanghai, A/Wisconsin, B/Phuket, and B/Washington. Briefly, plasma samples were first treated with enzymes to remove interfering sialic acid residues. MSD 96-Well 10-Spot multi-array plates coated with nine trimeric flu HA antigens (or no antigen/BSA control) were blocked then pretreated human plasma samples diluted 5,000-fold, along with a 1:4 dilution series of mixed human plasma positive controls and 2 or 4 wells of plasma-free negative controls (Blanks). Experimental plasma samples were plated in triplicate, while controls were plated in duplicate. Plates were shaken for 2 hours at 15°C to 25°C, then red blood cell vesicles labelled with electrochemiluminescent (ECL) SULFO-TAG were added. Plates were shaken for 2 additional hours at 15°C to 25°C, washed, then MSD GOLD Read Buffer B added and the plates were read on an MSD SECTOR S600 ECL plate reader. For each well, background signal reported by the no antigen control was subtracted from signal for antigen spots. Results are reported as percent inhibition relative to a no plasma diluent only control (Blanks). Samples with high inhibition of HA binding by red blood cell vessicles have low measured signal and therefore high percent inhibition relative to plasma-free controls where binding is not inhibited.